Perspective: The European Union is not prepared for geochemical carbon dioxide removal*
Negative emissions, carbon dioxide removal, minerals, enhanced weathering
Phil Renforth**
Papeles de Energía, N.º 28 (junio 2025)
The European Union has ambitious climate targets that may necessitate the removal of more than 500 million tonnes of carbon dioxide per year from the atmosphere by mid-century. A range of approaches have been proposed that attempt to accelerate natural rock weathering to promote “geochemical carbon dioxide removal” (gCDR), some of which are developing commercially though the voluntary carbon removal market. Progressive policy within the EU is on the verge of creating an incentive mechanism that could stimulate substantial expansion of gCDR activities, and there is world leading support for research and development to help underpin this policy agenda. Yet, there has been no systematic evaluation of the mineral resources within the EU for gCDR, or an exploration of the pathways to its efficient, equitable or cost-effective use. This manuscript makes a preliminary assessment of gCDR resources in across the EU’s member states and demonstrates a CDR potential on the order of 274 – 368 million tonnes CO2 per year based on the use of currently produced waste and by-product materials. The annual capacity could be further extended by 10’s-100’s million tonnes CO2 if extraction of appropriate rock was marginally scaled up in the coming decades. There is asymmetry of resource across the EU, which will create an uneven experience of costs and benefits if these technologies were deployed. Clearly, the EU has considerable potential for gCDR, and a systematic programme of evaluation is needed to map the resource, quantify potential reserves given the trajectory of the value of carbon removal, and employ systems level analysis such that the strengths of member states can be maximised through cooperation.
1. INTRODUCTION
Towards the end of 2024 the European Union (EU) published the Carbon Removals and Carbon Farming Regulation creating a voluntary framework for certifying carbon dioxide removal (CDR) (European Parliament and Council of the European Union, 2024). CDR includes a broad range of proposals (Committee on Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration et al., 2019) for removing carbon dioxide (CO2) from the atmosphere including managing biomass on the land surface (e.g., forestation) and underground (e.g., with regenerative agriculture), combusting biomass for energy while sequestering the produced CO2, or operating machines that extract CO2 directly from the air. CDR will form an important part of our response to climate change alongside rapid emissions reduction. The intergovernmental Panel on Climate Change anticipates CDR may be required to remove on the order of 10 billion tonnes (Gt) CO2 per year by 2050, with cumulative storage on the order of 500 GtCO2 by 2100 (Masson-Delmotte et al., 2022).
Geochemical CDR (“gCDR”, Campbell et al., 2022; Maesano et al., 2022) involves approaches that react atmospheric CO2 with naturally occurring rocks or anthropogenic alkaline materials. These include the addition of silicate minerals to agricultural land (Beerling et al., 2018) or coastal environments (Meysman and Montserrat, 2017), the reaction of alkaline wastes or by-products (steel slag, cement waste, mine waste) in engineered facilities (Renforth, 2019; Renforth et al., 2024; Stolaroff et al., 2005), and processes that produce reactive materials to increase ocean alkalinity (Kheshgi, 1995; Renforth and Henderson, 2017). Broadly, these approaches attempt to accelerate natural weathering, which helps to stabilize climate over millennia (e.g. Berner, 2001). The reactions can be simplified to those in Eq. [1-3] and show that the final repository of CO2 is either dissolved bicarbonate ions (HCO3, Eq. [1], [2] and [3]) or new solid minerals (e.g., calcium carbonate CaCO3, a combination of Eq. [1] or [2] and the reverse of [3]). Eq. [1] is an example of a reaction between a calcium silicate mineral, which are commonly found in basic and ultrabasic igneous rocks. Eq. [2]. Shows the reaction between calcium hydroxide, common in anthropogenic alkaline materials (cement, slag). Eq. [3]. Shows the reaction of a carbonate mineral, found commonly within carbonate rock (limestone, dolomite). HCO3- can be a long-term storage reservoir for carbon (>1000 years) if it resides in the ocean, the chemistry of which inhibits the reverse of Eq. [3] (Renforth and Henderson, 2017). Solid carbonate minerals are stable over millions of years.
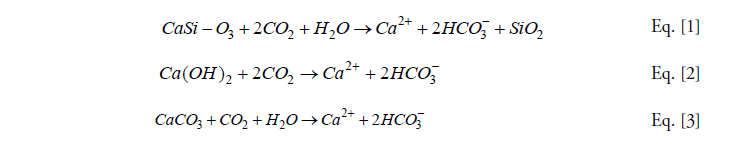
1.1. Mineral feedstocks
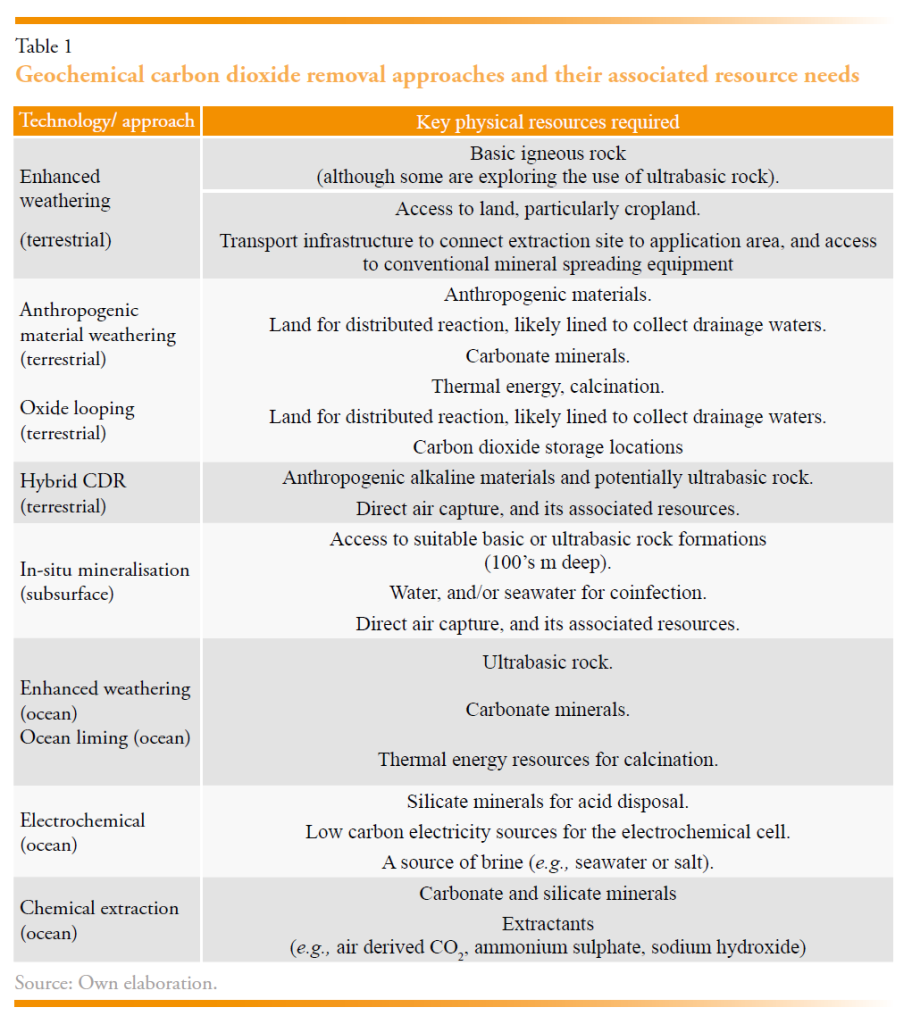
The above reactions also highlight the potential diversity of mineral feedstocks for gCDR. A summary of these is introduced below and is reviewed in greater detail by Lackner (2002). Indicative CDR potentials (in kgCO2 t-1) of these rocks are presented in Table 1. gCDR feedstock materials have been explored since the 1990’s for their ability to react with CO2 (Lackner et al., 1997), and specifically for reaction with atmospheric CO2 (Hartmann et al., 2013; Renforth et al., 2009; Schuiling and Krijgsman, 2006). Natural rocks are sufficiently abundant at the surface of the earth so their capacity for CO2 sequestration is not meaningfully constrained (Lackner, 2002). Based on contemporary production Renforth et al., (2011) estimated that anthropogenic materials might be about to react with ~1 GtCO2 yr-1, which may increase to 3-8 GtCO2 yr-1 for future production (Renforth, 2019), with a possible additional 1-4.5 GtCO2 yr-1 from mine waste (Bullock et al., 2021).
Basic igneous rocks are rich in magnesium and calcium but low in silica. They are typically dark-coloured, dense, and form from the cooling and solidification of magma or lava. Common examples include basalt, gabbro, and diabase. These rocks are abundant in oceanic crust and volcanic regions such as mid-ocean ridges and continental rifts. Mineralogically, basic rocks are dominated by plagioclase feldspar (typically calcium-rich varieties like labradorite) and pyroxenes (especially augite), with olivine often present in more ultrabasic forms. Chemically, they have high concentrations of iron, magnesium, and calcium. Upon metamorphism, they can transform into rocks like amphibolite or greenschist depending on pressure and temperature.
Ultrabasic rocks are igneous rocks with an extremely low silica content and are composed almost entirely of minerals rich in magnesium, iron, and calcium such as olivine and pyroxene. These rocks are typically dark, dense, and coarse-grained. Common examples include peridotite, dunite (mostly olivine), and pyroxenite (mostly pyroxenes), and typically contain accessory minerals like chromite, magnetite, and spinel. Ultrabasic rocks are found at the surface in ophiolite rock formations. When altered, ultrabasic rocks often form serpentine, talc, and chlorite, and they are important host rocks for nickel, chromium, and platinum-group metal deposits.
Carbonate rocks are sedimentary rocks primarily composed of carbonate minerals, most commonly CaCO₃ and dolomite (CaMg(CO₃)₂). These rocks form mainly through biological and chemical processes in shallow marine environments, often from the accumulation of shells, coral fragments, and other calcareous materials. The two main types are limestone, which is predominantly calcite, and dolostone (or dolomite rock), which is rich in dolomite. Carbonate rocks are typically light-coloured, relatively soft, and often contain fossils, reflecting their organic origin. Mineralogically, carbonate rocks are dominated by carbonate minerals but may also contain clay, quartz, or organic matter as impurities. Through metamorphism, they can transform into rocks such as marble, composed of recrystallized calcite or dolomite. Relatively pure calcite-rich limestones are raw materials for the cement and lime industries. The use of carbonate rocks as a feedstock for gCDR necessitates that the final repository of the formed bicarbonate is the ocean, formation of new solid carbonate minerals using these materials would not result in a net removal of CO2. Caserini et al., (2022) estimate that there are 5 trillion tonnes of limestone within 10 km of the coast globally.
Anthropogenic alkaline materials are substances that contain elevated concentrations of Ca, Mg, or Na, resulting in high pH on contact with water. They are primarily produced as byproducts or residues from industrial processes such as cement production, steelmaking, coal combustion, and mineral processing. Common examples include cement kiln dust, blast furnace slag, steel slag, fly ash, and red mud (a byproduct of aluminium refining). Mineralogically, alkaline materials are often complex and variable in composition but typically contain hydraulic and pozzolanic phases such as portlandite (Ca(OH)₂), oxides (CaO, MgO), calcium sulphate phases, calcium silicate hydrates (C-S-H), and glassy aluminosilicates.
1.2. Geochemical carbon dioxide removal
Geochemical CDR approaches facilitate reaction with carbon dioxide by either increasing the reactivity of a material (e.g., through the reduction of particle size, and increase in reactive surface area, (Renforth, 2012; Tromans, 2008)) and/or placing the material into a more reactive environment (e.g., a reactor with elevated CO2 (Gerdemann et al., 2007), elevated CO2 injected into reactive rock formations (Matter et al., 2016), soil (Beerling et al., 2018), or the ocean (Renforth and Henderson, 2017). These approaches are reviewed by Campbell et al. (2022), Eisaman et al. (2023), and Kelemen et al. (2020), but have been summarised below. The key resources required for each approach is presented in Table 1.
1.2.1. Terrestrial gCDR approaches
Kelemen et al. (2020) propose three categories of approaches for reacting minerals with CO2. “Surficial CDR” involves the above ground handling and reaction of minerals distributed over large areas. The second category, “In-situ CDR”, involved the injection of CO2 into reactive rock formations. Finally, “hybrid CDR” involve the combination of direct air capture and mineralisation.
Surficial CDR can be further characterised as either reacting minerals in soils or the landscape (enhanced weathering), the reaction of anthropogenic materials (e.g., mine wastes) in controlled facilities, or the engineered production of reactive materials for oxide looping CDR.
Enhanced rock weathering (ERW) is typically implemented by spreading finely ground silicate rocks (such as basalt) over croplands, where weathering reactions with CO₂ in soil water produce stable dissolved bicarbonate ions that can be transported to oceans (Hartmann et al., 2013; Schuiling and Krijgsman, 2006). A compelling benefit of ERW is its synergy with agriculture, it potentially improves soil pH, releases beneficial nutrients, and increases crop yields while capturing CO₂ (Beerling et al., 2018). Empirical field trials in the U.S. Corn Belt have demonstrated net CO₂ removals of over 15 tons per hectare over four years, coupled with up to 16% yield increases in staple crops (Beerling et al., 2024). In the EU, Carbon Neutral Initiative (Netherlands/Spain), ClimeRock (France), Greensand (Netherlands), Green Sequest (Poland), Silicate (Ireland), The Rock Flour Company (Denmark), and ZeroEx (Germany) are examples of commercial ERW projects.
Weathering and carbonation of anthropogenic materials. Anthropogenic alkaline materials (cement, slag, ash, mine tailings) are well known to react with atmospheric CO2. The formation of solid carbonates is consistently observed in and around legacy deposits of slag (Mayes et al., 2018; Renforth et al., 2009) lime (Andrews et al., 1997), and mine tailings (Wilson et al., 2014, 2009) and is common in brownfield soils containing cement from demolition waste (Renforth et al., 2009; Washbourne et al., 2015). Kelemen et al., (2020) suggests that it may be possible to carbonate the most reactive components of these waste materials (e.g., Mg(OH)2 or Ca(OH)2) by passively, or through limited mechanical mixing, distributing the material in thin layers over large areas. Arca operating in Canada and Australia is a commercial example of this approach.
Oxide looping involves reacting hydroxide minerals with atmospheric CO2. It is possible to produce oxide/hydroxide materials at industrial scale (globally >400 Mt of lime are produced annually (Apodaca, 2025)) to react with atmospheric CO2 (see McQueen et al., 2020). In this process, carbonate minerals (MgCO3, CaCO3) are calcined at elevated temperature to produce oxides (MgO, CaO) which are hydrated to hydroxide minerals (Mg(OH)2, Ca(OH)2). Spreading these hydroxide minerals into heaps exposed to atmospheric CO2, would result in their carbonation (MgCO3, CaCO3). If the emissions from the production process were substantially reduced (e.g., through carbon capture and storage), then there would be a net removal of CO2 out of the atmosphere into carbonate minerals. Recycling the carbonate minerals as a feedstock into calcination would essentially transfer CO2 from the atmosphere into the flue gas of the kiln. Calcite/8 Rivers (United States), Heirloom (United States), and Origen (UK) are developing this technology.
Hybrid CDR involves the combination of ex-situ mineralisation with elevated CO2 provided by direct air capture or biogenic sources. Ex-situ mineralisation was initially proposed as a method to sequester CO2 produced from point sources of emissions (Lackner et al., 1995; Seifritz, 1990). In these processes, CO2 would be captured and purified from a flue gas and then reacted with water and minerals at elevated temperatures and pressures. Ultrabasic rocks (given their faster reaction kinetics) were explored as the primary feedstock, in which they would need to be extracted and finely ground (<100 µm). Gerdemann et al., (2007) suggest optimum reaction conditions of >150°C and pCO2 > 100 bar. Faster reaction times, under lower temperatures and pressures has been reported for other naturally occurring (wollastonite, Gerdemann et al., 2007; Huijgen et al., 2006) and anthropogenic (steel slag, Huijgen et al., 2005; cement kiln dust, Huntzinger et al., 2009) materials. The energy-intensive nature of mineral pre-treatment (e.g., grinding and thermal activation) and the capital costs of operating a reactor at elevated pressures for slow reaction kinetics, have limited its deployment at scale (Metz and Intergovernmental Panel on Climate Change, 2005). Particularly, it performs poorly in competition with relatively inexpensive disposal of CO2 underground. However, integration of mineralisation within the cement sector may offer opportunities for direct ex-situ mineralisation to become competitive (Bremen et al., 2022). Indirect mineralisation was proposed to overcome limitations in the reaction kinetics by dissolving or extracting the magnesium out of the rock using a strong extractant (e.g., a strong acid or base) before reacting it with CO2 (e.g., Wang and Maroto-Valer, 2011; Zhang et al., 2010). These alkaline materials may be directly reacted with atmospheric CO2 (Madeddu et al., 2015) or coupled to direct air capture (Ragipani et al., 2022). In the EU, Blue Skies Minerals (Germany), Carbonaide (Finland), Carborok (France), ecoLocked (Germany), and Paebbl (Netherlands/Sweden) are commercial projects developing Hybrid CDR technologies.
In-situ mineralisation refers to the process of injecting CO₂ derived from direct air capture into reactive subsurface geological formations, where it naturally reacts with silicate minerals to form stable solid carbonates (Matter et al., 2016). Successful field-scale demonstrations, such as the CarbFix project in Iceland, have proven that mineralisation can occur rapidly (within years). The project dissolving CO₂ in water before injection to enhance mineral reactivity and minimise leakage. As CO2 is more soluble at higher pressures, the co-injection of water becomes less feasible with shallower injection depths. CarbFix injected into rock at depths of 400-800 m, which corresponds to a hydrostatic pressure of 40-80 bar. At these pressures 10’s tonnes of water are required for every tonne of CO2. At <50m that would correspond to 100’s of tonnes of water per tonne of CO2 (Snæbjörnsdóttir et al., 2020). The application of in-situ mineralisation to the EU will require rock formations at suitable depth, with access to fresh water, and potentially seawater. 44.01 (Oman), CarbFix (Iceland), and Cella (Kenya) are commercial in-situ mineralisation projects.
1.2.2. Ocean alkalinity enhancement approaches
Ocean Alkalinity Enhancement (OAE) is a gCDR strategy that involves adding alkaline substances (like crushed minerals or alkaline solutions) to the ocean. This process enhances the ocean’s ability to absorb and store atmospheric CO₂ as dissolved bicarbonate and carbonate ions. It could also help to ameliorate ocean acidification. OAE approaches are reviewed in Renforth and Henderson (2017) and Eisamann et al., (2023). See Oschlies et al., (2023) and references therein for considerations on the broader environmental and social implications of OAE. The taxonomic classification in Lee Pereira et al., (2025) has been simplified and adapted below.
Coastal enhanced weathering involves spreading ultrabasic rock onto coastal environments (Meysman and Montserrat, 2017; Montserrat et al., 2017), in which wave action, lower pH of surface sediments, and rapid replenishment of coastal waters is thought to accelerate mineral dissolution (Schuiling and de Boer, 2010). Experiments suggest that high olivine concentrations in the sediment could result in a reduction in alkalinity (Fuhr et al., 2021), suggesting care should be taken when distributing the material. Collisions between particles, e.g., through wave action, may also accelerate the dissolution and reaction of the mineral (Flipkens et al., 2023). Vesta (United States) is commercially developing this technology.
Ocean Liming involves the intentional addition of alkaline materials, particularly hydrated lime (Ca(OH)2), to seawater (Kheshgi, 1995; Renforth et al., 2013). In this process, carbonate minerals are used as the feedstock and transformed through calcination and hydration into Ca(OH)2. The hydrated lime is added to the ocean via ships. For this process to contribute to CDR, the emissions at the lime kiln need to be substantially reduced (Foteinis et al., 2022). Rapid mixing within the wake of a ship quickly dilutes the added alkalinity (Caserini et al., 2022). Limenet (Italy) are exploring the commercialisation of a variant of ocean liming.
Electrochemical approaches involve splitting seawater or brine into acidic and alkaline streams using electrodialysis (Eisaman, 2024) or membrane electrolysis (Rau et al., 2013), followed by managing or neutralizing the acidic by-products and returning the alkaline-enriched water to the ocean. The elemental reservoir of the ocean is practically unlimited for electrochemical approaches, but pretreatment of the seawater or upgrading its salinity pose practical challenges to its implementation. The high electricity demand for electrochemical systems, means that they will only be effective for CDR if they operate on low-carbon electricity sources (Committee on Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration et al., 2019). Ebb Carbon (United States) are commercialising a method of electrochemical OAE.
Enhanced extraction techniques include a range of treatments to create more reactive forms of alkalinity that can be added to the ocean. These include digesting carbonate rocks under elevated CO2 derived from DAC or biomass (Rau, 2011), high pH digestion of ultrabasic silicate rock (Madeddu et al., 2015), the conversion of carbonates into hydrated carbonates (Renforth et al., 2022) or extraction through ammonium sulphate (Nduagu et al., 2012). Calcarea (United States) are commercialising a method of carbonate rock digestion under CO2 rich flue gases onboard ships, Cambridge Carbon Capture (UK) are commercialising a method of alkaline digestion of silicates, and the Planeteers (Germany) are exploring hydrated mineral carbonate OAE.
1.3. Climate change mitigation in the European Union
The EU is a global leader in climate change mitigation, guided by an ambitious policy framework and robust research agenda. At the centre of EU climate policy is the European Green Deal, aiming to make Europe a net-zero continent by 2050. The European Scientific Advisory Board on Climate Change, established in 2021 by the European Climate Law, provides independent advice to the EU. Their 2025 report Scaling up Carbon Dioxide Removals suggests 544–568 MtCO2 yr-1 will be required by 2050 to help meet EU climate policy targets. In their view, this could be met through a combination of land use, biomass energy carbon capture and storage, and direct air capture (European Scientific Advisory Board on Climate Change, 2025). The report simplifies gCDR into enhanced weathering and ocean alkalinisation, highlighting opportunities and risks in the implementation, but acknowledging that the field is at a relatively early stage of development. A similar approach was adopted by the equivalent organisation in the UK (Climate Change Committee), but in their most recent advice to government, The Seventh Carbon Budget, they now include an 8% contribution to 2050 CDR targets using technologies that include enhanced weathering (Climate Change Committee, 2025).
The EU offers world leading support for CDR research, with several large projects funded through a range of programmes (Table 2). In addition, the EU supported the CarbFix2 project (2017-2021, €2.2M), initially proposed to store industrial CO2, has subsequently been commercialised as a repository for atmospheric CO2. Carbon Gap estimates that the EU has allocated €657M to support CDR methods between 2020 and 2023 and suggest that this will need to increase to €3-6 billion over the next 15-20 years (Carbon Gap, 2025a).
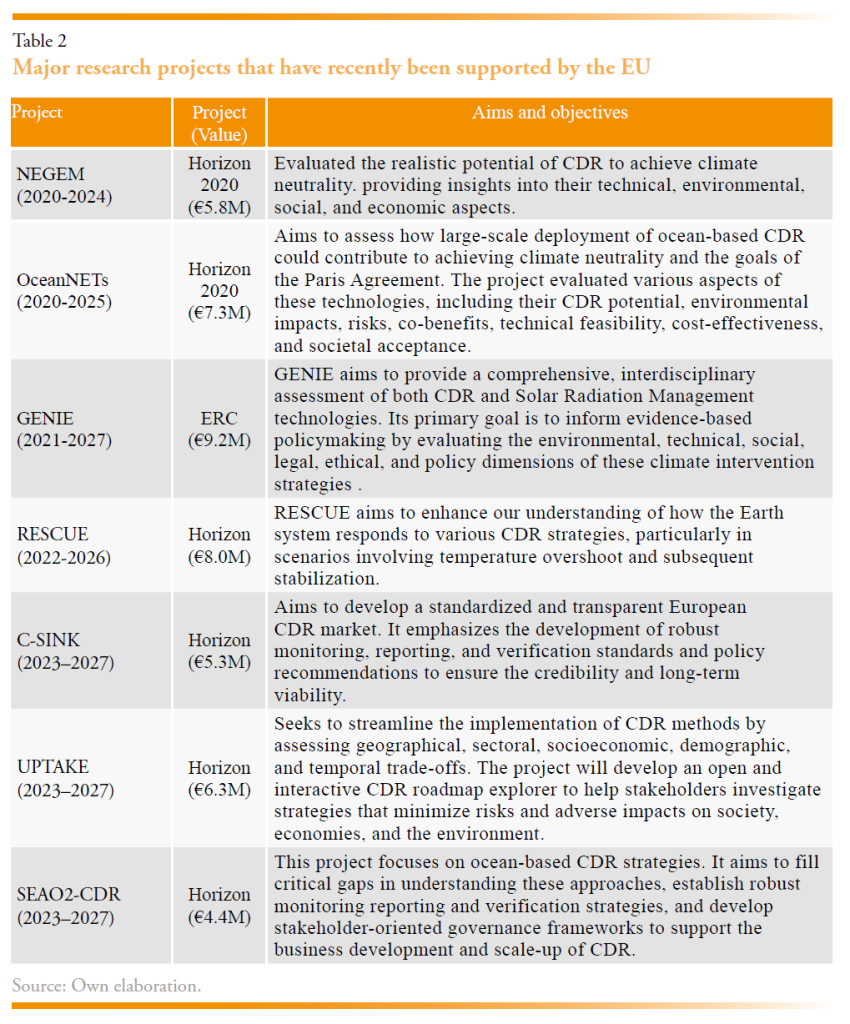
Despite this investment, there is no robust overview of the EU’s geological resources for gCDR, nor pathways for exploiting this resource. Such an assessment is essential to identify the barriers to gCDR scale up, to understand how resources may be efficiently managed, what technologies are nationally relevant and those that require EU-wide policy, or how the cost and benefits may be shared amongst member states.
Previous work in the UK has mapped some gCDR resources (Madankan and Renforth, 2023; Renforth, 2012), explored the potential of enhanced rock weathering (Kantzas et al., 2022), and paired resources to application sites to explore realistically constrained supply chains (Madankan et al., in review), see Section 3. The aim of this work is to provide an initial perspective on the gCDR resources in the EU, summarise potential barriers and opportunities for developing those resources, and suggest further work that is required to create meaningful climate CDR policy for this technology.
2. RESOURCES IN THE EUROPEAN UNION FOR GCDR
2.1. Resource deposits
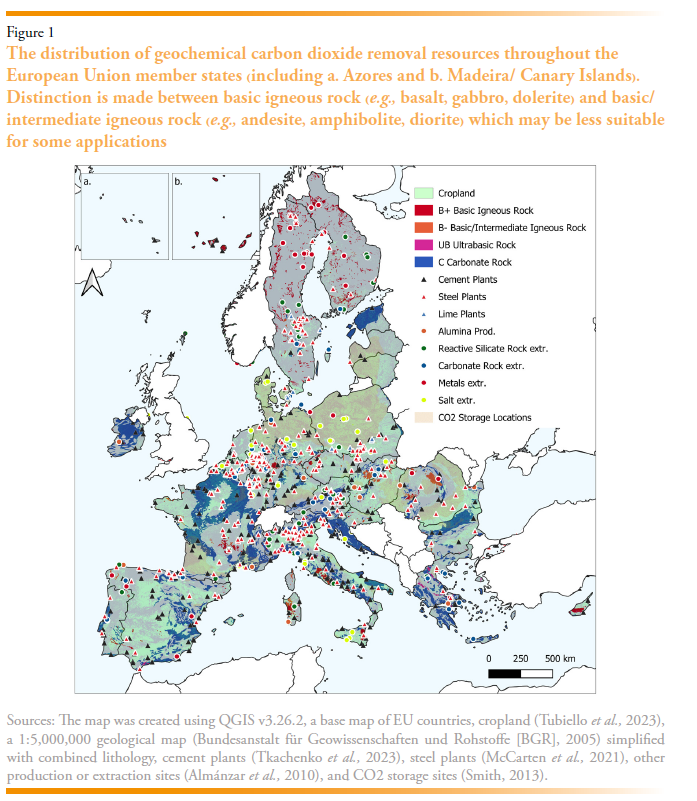
The EU possesses significant natural and anthropogenic mineral resources that can support scalable and durable gCDR. A summary of the natural rock resources and a brief national overview is included in Table 3 for EU member states, and their spatial distribution is presented in Figure 1. While the carbonate rocks are largely abundant across most member states, limited carbonate resources are present in Denmark, Finland, and the Netherlands. The most suitable basic rock resources are less widely distributed, situated in only 13 of the member states. Ultrabasic rock deposits are present in only 9 of the member states.
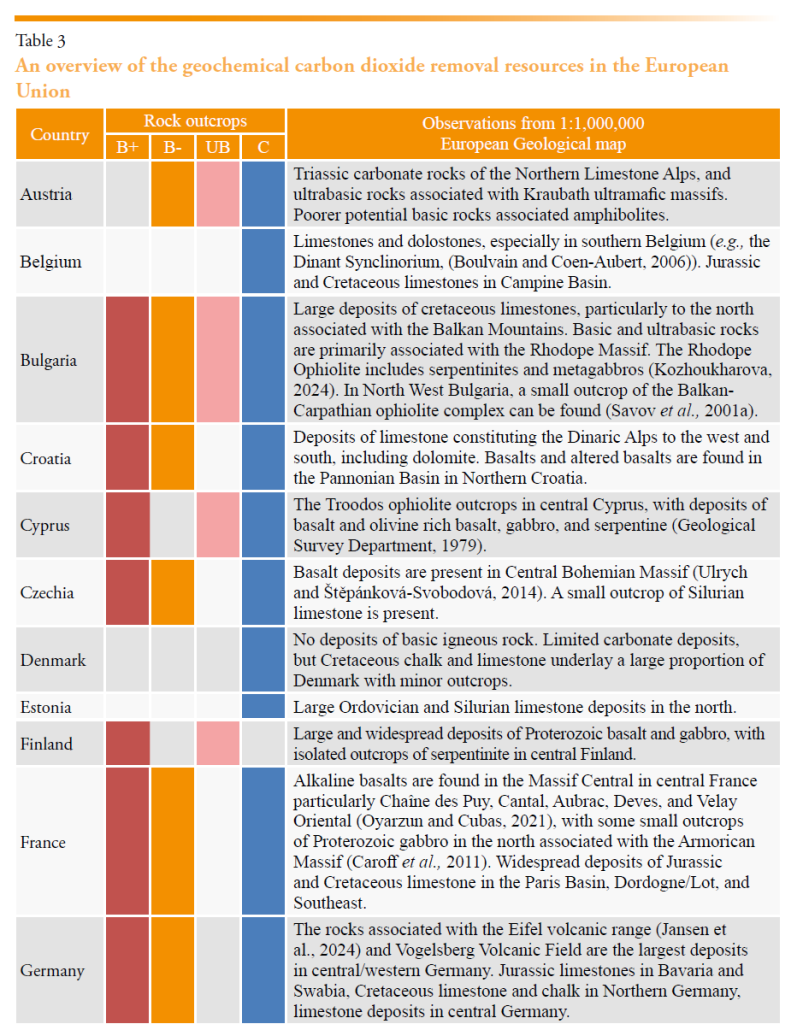
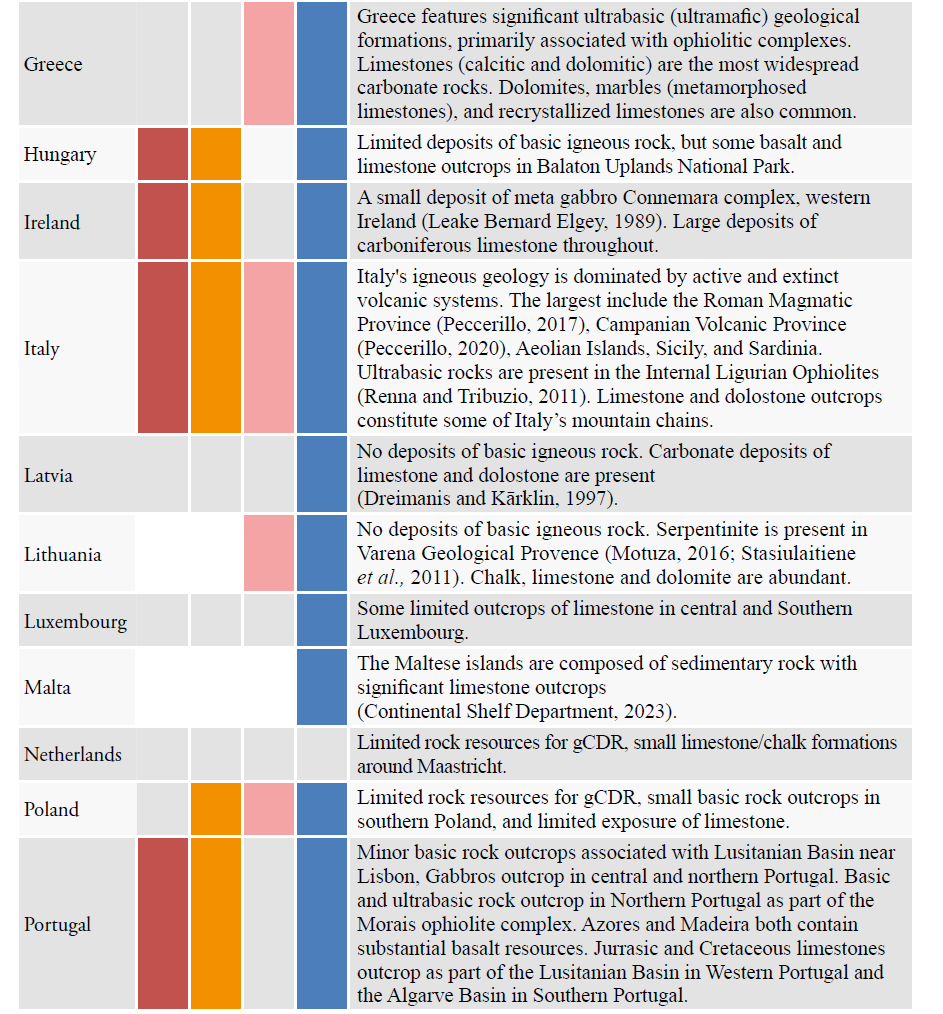
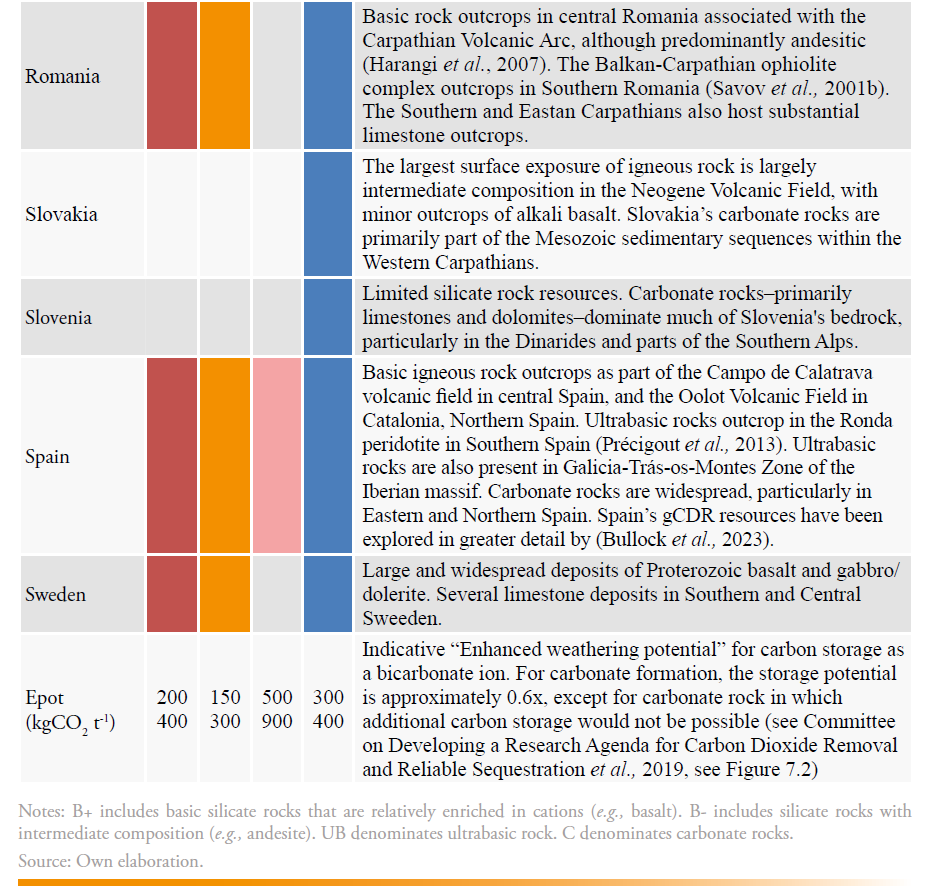
The information in Table 3 was synthesized from observations made using a geological map (Bundesanstalt für Geowissenschaften und Rohstoffe (BGR), 2005) with a resolution of 1:5,000,000, and supported with additional literature where possible. It is not exhaustive, and it could miss smaller yet volumetrically significant resources. There is no consideration of what might be practically extracted, or what social or environmental restrictions may be in place for these deposits.
2.2. Annual production of resources
The extraction and transformation of geological resources that have relevance for gCDR have been summarised in Table 3 including crushed rock, salt, cement, lime, and steel, with their spatial distribution shown on Figure 1.
The lithology of crushed rock production is rarely reported and the information in Table 3 represent the combined value for sedimentary (e.g., limestone, sandstone) and igneous (basic, intermediate, or acidic) rocks. Such distinction is mostly unnecessary for their primary purpose of construction aggregate, yet it is essential for understanding the current capacity and scale-up potential of gCDR. The EU extracts approximately 1.2 Gt of crushed rock each year. Approximately 25% of this material will be waste “fines” <1-5mm (Mitchell, 2009), equating to around 400 Mt. Not all this material will be applicable for gCDR, but assuming 50% is carbonate rock (300 kgCO2 t-1) and 10-20% is basic igneous rock (200-400 kgCO2 t-1) then this waste material may be able to contribute 60 MtCO2 y-1 and 8-32 MtCO2 yr-1 of CDR for carbonate and basic igneous rock respectively.
The cement and lime industry have three mechanisms by which they might contribute to CDR. First, cement production creates waste or by-product kiln dust, equating to around 115 kg t-1 (Huntzinger et al., 2009), which can react with CO2. Second, cement and some lime materials may already react with atmospheric CO2 during their service life (Xi et al., 2016) or have the potential to react post-use (Washbourne et al., 2015). Finally, spare capacity within kilns, reported to be 40-50% in the EU (Harder, 2023), could be used to manufacture lime for ocean liming (Kheshgi, 1995; Renforth et al., 2013) or direct air capture (Erans et al., 2020; McQueen et al., 2020). The later two of these mechanisms requires deep emissions reduction at the production site for net removal (Foteinis et al., 2022). Collectively, the EU produces around 135 Mt of cement and lime annually. Approximately 20% of current lime production applications result in a reaction with CO2 and approximately 15% of cement may already react with atmospheric CO2 during its service-life (see Renforth, 2019 and references therein) resulting in 12 MtCO2 yr-1 removed from the atmosphere. Around 115 kg of cement kiln dust is produced per tonne of cement clinker, this could capture a further 4.2 Mt CO2 yr-1. The chemical decomposition of limestone represents >50% of the emissions in a cement plant, the equivalent mass of CO2 may be recaptured if cement were carbonated following use (e.g., during demolition). This could equate up to 61 MtCO2 yr-1 based on contemporary production. Cement kilns within the EU operate below capacity (some suggesting 60% (Harder, 2023)), if this were used to produce lime for either direct capture of atmospheric CO2 (Erans et al., 2020; McQueen et al., 2020) or for ocean liming (Renforth et al., 2013), that would result in an additional 63-82 MtCO2 yr-1 CDR, although the emissions from the production site would need to be substantially reduced for this to remove more CO2 than emitted.
By-product and waste slag from the steel industry is also known to react with atmospheric CO2 in legacy deposits (Mayes et al., 2018; Pullin et al., 2019; Renforth et al., 2009). Accelerating this reaction with atmospheric CO2, while simultaneously reducing steel greenhouse gas emissions, could result in a net negative steel industry (Renforth et al., 2024). Approximately 140 Mt of steel is produced by the EU annually, creating 42 Mt of slag. This has the potential to react with 16 MtCO2 yr-1.
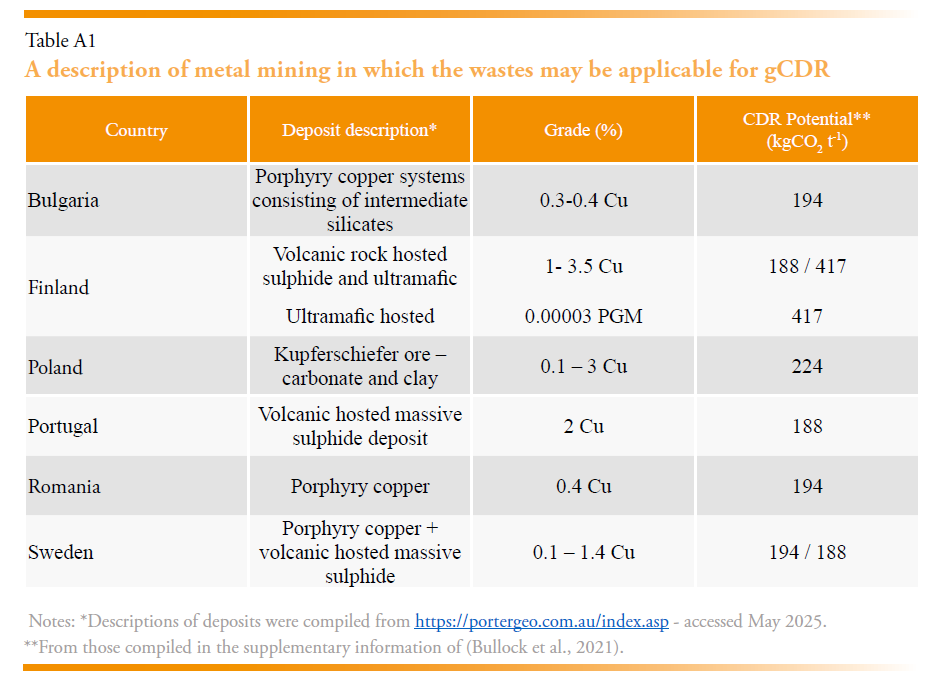
There are several waste materials produced from mining that may be appropriate for geochemical CDR (Bullock et al., 2021). Chromium (Cr), copper (Cu), nickel (Ni), and platinum group metals (PGM) are the most significant for the EU, the typical commercial ore grades range from 2-25% (Cr), 0.2-3% (Cu), 0.2-2% (Ni), 3 x10-5% (PGM). The remaining “gaunge” is composed of minerals that could be potentially used as a feedstock for gCDR technologies. In addition, the processing of bauxite for aluminium creates “red mud”, a hyper alkaline waste (Renforth, 2019). The CDR potential of mine waste is dominated by waste from Cu mining in Bulgaria, Finland, Poland, Portugal, Romania, Spain and Sweeden. Table A1 provides an overview of the deposit types and their potential for CDR. Simplifying in Table 4, the potential for mine waste weathering may be on the order of 75 – 132 MtCO2 yr-1, although this is an initial estimate and could be substantiated with chemical analysis of the specific waste deposits.
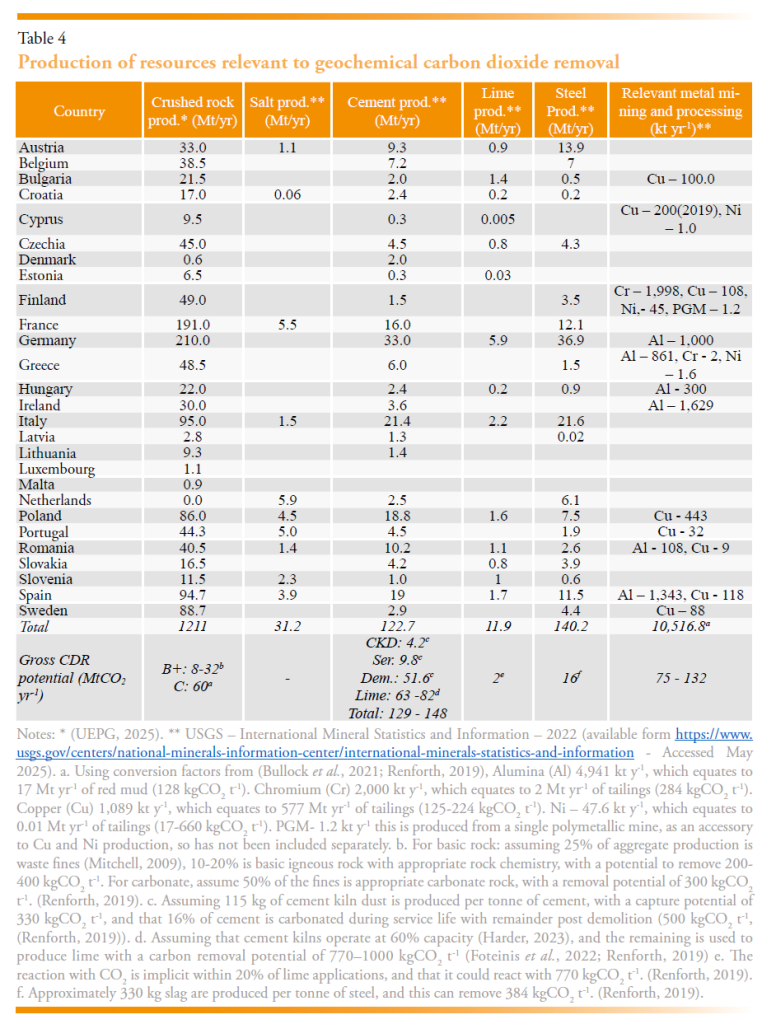
Salt production is noted in Table 3 given that some approaches to ocean alkalinity enhancement propose to use electrochemistry to split salt into acid and base streams (Eisaman, 2024; Rau, 2008; Rau et al., 2013). In these technologies the base stream is added to the ocean, resulting in an increase in ocean alkalinity and a removal of CO2 from the water, and eventually the atmosphere. The acid stream from this process would need to be neutralised by reaction with a silicate rock to operate these approaches at climate relevant scales. Approximately 31 Mt of salt are extracted and consumed annually in the EU. If electrochemical processes were to exploit mined salt to create alkalinity, at least 1.3 t salt would be required for every tCO2. Most of the proposed electrochemical approaches use seawater as the source of brine (Eisaman, 2024; Eisaman et al., 2023).
The total gross CDR potential for waste materials or by-products in the EU is approximately 124-42 MtCO2 yr-1, and an additional 12 MtCO2 yr-1 could already be occurring due to the reaction of lime and cement with CO2 during their service life. If the emissions at cement kilns were net-zero, and that the spare capacity that is present in the EU were used to produce lime for CDR, then an additional 63-82 MtCO2 yr-1 removal potential could be realised. Finally, the possibility of using mineral wastes from metal mining could contribute an additional 75–132 MtCO2 yr-1, the efficacy of which depends on the geology of the host rock. The total resource potential based on contemporary production is 274–368 MtCO2 yr-1. Upscaling rock extraction specifically for gCDR would add additional resource. For instance, a 20% increase in current rock extraction in materials that are relevant to gCDR with a capture potential 200-400 kgCO2 t-1, would equate to an additional 48-97 MtCO2 yr-1 of CDR capacity.
3. NATIONAL CASE-STUDIES
There is limited exploration of gCDR for nation states, which have been summarised below. While these studies are useful for demonstrating potential and shaping policy, they do not capture nuances of resource sharing through cooperation, a strength of the EU’s internal market.
3.1. Enhanced Rock Weathering in the UK
Enhanced rock weathering (ERW) is one of the more widely known methods of gCDR, which involves the distribution of crushed rock onto agricultural fields (Hartmann et al., 2013; Schuiling and Krijgsman, 2006). Model simulations have shown substantial scalable potential at competitive cost, with potential secondary benefits to farmers (Beerling et al., 2020), which has catalysed early-stage investment and deployment.
Deployment of ERW within the UK has received considerable attention. Initially Renforth (2012) mapped ERW resources in the UK and showing that their total capacity for CO2 sequestration was >400 GtCO2, which is much greater than any realistic future need. The calculated costs encompassed a substantial range (€2024 71-590), largely controlled by uncertainty in the mineral weathering rate. Kantzas et al., (2022) implemented a geochemical weathering model coupled with a cost assessment to show that ERW may cost €2024 102-139, scale to 6-30 MtCO2 yr-1 (the higher end of the range being equivalent to >40% of anticipated UK CDR requirements). Madankan and Renforth (2023) refined the UK resource assessment, identifying 68 current basic igneous rock production sites, their production volumes equated to around 14 Mt yr-1, and that planning permission has been granted to extract 490 Mt. Finally, Madankan et al., (in review) has explored extraction scenarios and demonstrated that for large scale deployment in the UK, a large flow of material across the country from production sites to agricultural land would be required. This work has led to the introduction, albeit without adoption, of ERW in UK climate policy (UK Government, 2021), and the recent advice to UK Government on the implementation of enhanced weathering into their net zero pathway (Climate Change Committee, 2025).
The global assessment of Beerling et al., (2020), simulated the potential of ERW in France, Germany, Italy, Poland, Spain using their weathering model, and estimate a collective potential for 56-206 Mt CO2 removal, for applications between 10-50% of cropland. This model predicts an annual removal rate of approximately 5.1-6.6 tCO2 ha-1.
It is challenging to obtain empirical data to validate these models given that field experiments must be undertaken over several growing seasons, across a range of soils, crops, climates, and mineral addition rates, and that appropriate protocols for measurement are still under development (Clarkson et al., 2024), with mixed results reported for smaller scale experiments (Buckingham et al., 2022; Reershemius et al., 2023). However, measurements from field experiments suggest that annual removal rates of 1.3 - 2.6 tCO2 ha-1 yr-1 may be possible (Beerling et al., 2024; Larkin et al., 2022). The first credits issued for ERW by Isometric to InPlanet for a 500 ha addition in Brazil suggests a removal rate of approximately 1.1 tCO2 ha-1 yr-1 (although only 36% of that was claimed in the credit (Isometric, 2025)).
A simple estimate for the potential of ERW in the European Union is presented in Table 5 assuming an annual removal rate of 1 tCO2 ha-1 and applied to 25-75% of national cropland. This could result in 34 to 100 MtCO2 yr-1, of which France, Germany, Italy, Poland, and Spain would contribute 70% of this potential. Even the lower estimate is significant in the context of a possible EU target of 500 MtCO2 yr-1 by mid-century. The total basic igneous rock requirement, assuming an application of 30 t ha-1 would be on the order of 1.3 to 4.0 Gt yr-1. The lower estimate is equivalent to total current annual rock production in the EU, and an unquantified multiple of current basic igneous rock extraction. While the scale-up of rock production would be challenging, potentially 6-24 MtCO2 yr-1 could be met using waste quarry fines (Table 3), and Madanken et al., (in review) has shown that an equivalent scale up in the UK is plausible over decades. Table 3 highlights the asymmetry that would exist in Europe between member states in which rock is produced and where it may be applied. For instance, a lack of resource availability in Northern (Belgium, Denmark, Estonia, Latvia, Lithuania, Luxembourg, Netherlands, and Poland), Eastern (Slovakia), and Southern Europe (Greece, Malta, Slovenia), and small deposits in Ireland, Hungary and Portugal could limit 30% of removals from ERW.
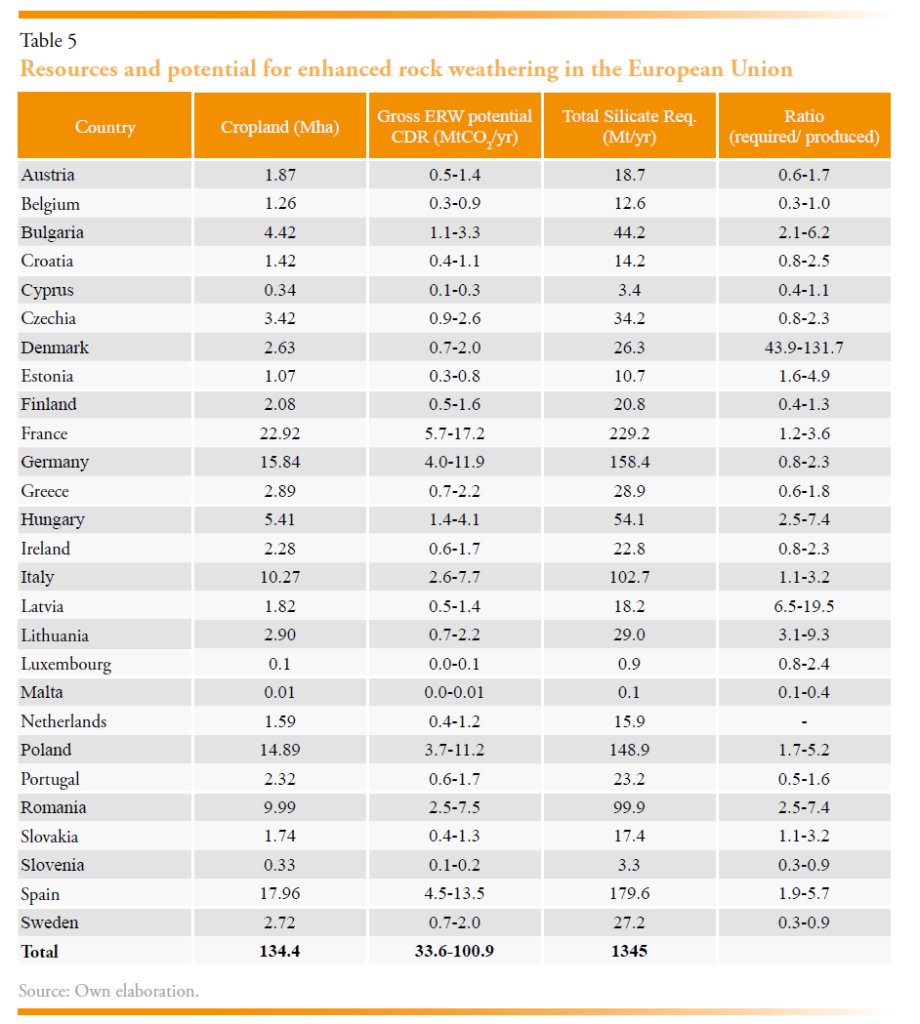
Barriers to implementing ERW include the logistical challenge of scaling up the supply chain for mineral distribution across diverse EU agricultural lands (Oppon, 2020). Additionally, policy and regulatory uncertainty around land use, environmental consequences at large scale, and carbon accounting may slow development (Clarkson et al., 2024; Spence et al., 2021; Steg et al., 2022). Systems for monitoring ERW are at an early stage and lack long-term field data that would provide confidence to the market (Clarkson et al., 2024). Lastly, there are public acceptance issues, driven by limited awareness and scepticism (Spence et al., 2021). Overcoming these barriers will require integrated policies, targeted subsidies, streamlined regulatory frameworks, and investment in monitoring technologies. Critical to this is a systematic assessment of ERW resources and their deployment patterns to agricultural land.
3.2. Geochemical CDR in Spain
Spain’s National Energy and Climate Plan sets out a 23% reduction in greenhouse gas emissions by 2030 (Braga et al., 2022) with a 2050 target of 90% reduction (Sun et al., 2021). Currently Spain’s long term climate strategy aims for “natural” removals absorbing the remaining using a combination of reforestation, wetland restoration, and agroforestry (equivalent to 37 MtCO2 yr-1 by 2050, (Carbon Gap, 2025b).
Spain has approximately 14,800 km2 of basic and ultrabasic rock near to the surface, with around 1,900 km2 of exposed material in unprotected areas (Bullock et al., 2023). The latter would equate to approximately 12.7 Gt of material (assuming a 20 m quarry working depth, and a rock density of 3 t m-3), with a CDR potential of 5 GtCO2. Similar to the UK (Renforth, 2012) this is a resource considerably greater than any realistic future needs of Spain. Similarly, carbonate rock deposits cover approximately 109,500 km2, and around 773 km2 that are exposed in unprotected areas. Industrial sector by-products may be able to remove an additional 7.7 MtCO2 yr-1 (Bullock et al., 2023). gCDR could make a significant contribution to Spain’s future CDR requirements.
An exploration of ocean alkalinity deployment scenarios for Spain, Foteinis et al., (in review) take a prospective life-cycle assessment approach for examining 3 technologies (ocean liming (Renforth et al., 2013), coastal enhanced weathering (Meysman and Montserrat, 2017), and electrochemistry (Eisaman, 2024). This case study specifically explores opportunistic use of existing industries and supply chains (e.g., mining, calcination, and desalination) to deploy these technologies, and suggests that a net CDR potential of >9 MtCO2 yr-1 is possible by 2030, increasing to >40 MtCO2 yr-1 by 2050.
Deployment of ocean alkalinity enhancement in the EU may be constrained by scientific, legal, economic, and societal barriers. One of the foremost scientific challenges is the uncertainty in carbon sequestration efficiency and short- and long-term ecological effects, which complicates regulatory approvals and public support (Oschlies et al., 2023). Furthermore, governance gaps within the EU and internationally, especially in the application of the London Protocol, limit legal clarity on the permissibility and liability of large-scale ocean alkalinity enhancement (Webb et al., 2021). Economically, the high cost and energy intensity of alkaline material production and their distribution limit implementation of some approaches (Eisaman et al., 2023). Furthermore, the lack of standardised monitoring, reporting, and verification protocols creates a lack of confidence in the technologies ability to integrate with climate markets. Low public awareness and stakeholder scepticism toward marine based CDR limit political momentum and investment (Lezaun and Valenzuela, 2024). Overcoming these barriers will require a coordinated EU-wide policy framework, robust pilot studies, and public engagement strategies.
4. CONCLUSION
Geochemical CDR approaches are emerging as promising methods to remove carbon dioxide from the atmosphere. While these approaches have not penetrated mainstream climate policy in the EU, they are beginning to be considered as part of the UKs CDR portfolio. The analysis above suggests that current production of by-product or waste mineral resources in the EU has the potential to remove 274 – 368 MtCO2 yr-1. This potential is distributed between aggregate fines, alkaline wastes (cement kiln dust, slag), and metal mining waste. It also includes what may already be occurring through in-service life carbonation of concrete and lime, and the potential to leverage spare capacity within the cement sector to produce additional alkaline materials. The latter of which would require substantial reduction in emissions at cement production sites for this to be net CDR. Further expansion of rock extraction could add to this capacity, for instance an additional 34-101 MtCO2 yr-1 might be contributed through enhanced weathering. These quantities are significant in the context of the expectation that the EU may need to remove 550 MtCO2 yr-1 by 2050.
There is considerable asymmetry of resources across the EU. For instance, limited appropriate rock may limit enhanced weathering deployment in Poland, unless resources are transferred from other EU member states. Similarly, the deployment of ocean alkalinity enhancement will only be possible from coastal regions, potentially stranding limestone resources in Central and Eastern Europe unless low-cost, low carbon transportation were used. This asymmetry will influence deployment patterns of specific gCDR technologies and will influence how benefits and burdens are distributed.
The EU urgently requires a systematic evaluation of gCDR resources. There is a wealth of in country knowledge, particularly embedded within national geological surveys, that could be harnessed to provide an inventory of resources. In addition, a systems level analysis would establish the potential pathways for exploiting these materials, and the level of incentives needed to stimulate gCDR commercial projects. Without this, the EU is at risk of creating incentives that promote inefficient use of the resource, and it would certainly be unable to plan for long-term development.
APPENDIX
References
Almánzar, F., Baker, M. S., Elias, N., Guzmán, E. (2010). Mineral Facilities of Europe. U.S. Geological Survey, Reston, VA. https://doi.org/10.3133/ofr20101257
Andrews, J. E., Gare, S. G., Dennis, P. F. (1997). Unusual isotopic phenomena in Welsh quarry water and carbonate crusts. Terra Nova, 9, 67–70. https://doi.org/10.1111/j.1365-3121.1997.tb00004.x
Apodaca, L. E. (2025). Mineral Commodity Summaries 2025 – Lime. U.S. Geological Survey, Reston, VA. https://doi.org/10.3133/mcs2025
Beerling, D. J., Epihov, D. Z., Kantola, I. B., Masters, M. D., Reershemius, T., Planavsky, N. J., Reinhard, C. T., Jordan, J. S., Thorne, S. J., Weber, J., Val Martin, M., Freckleton, R. P., Hartley, S.E., James, R. H., Pearce, C. R., DeLucia, E. H., Banwart, S. A. (2024). Enhanced weathering in the US Corn Belt delivers carbon removal with agronomic benefits. Proceedings of the National Academy of Sciences, 121, e2319436121. https://doi.org/10.1073/pnas.2319436121
Beerling, D. J., Kantzas, E. P., Lomas, M. R., Wade, P., Eufrasio, R. M., Renforth, P., Sarkar, B., Andrews, M. G., James, R. H., Pearce, C. R., Mercure, J. -F., Pollitt, H., Holden, P. B., Edwards, N. R., Khanna, M., Koh, L., Quegan, S., Pidgeon, N. F., Janssens, I. A., Hansen, J., Banwart, S. A. (2020). Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature, 583, 242–248. https://doi.org/10.1038/s41586-020-2448-9
Beerling, D. J., Leake, J. R., Long, S. P., Scholes, J. D., Ton, J., Nelson, P. N., Bird, M., Kantzas, E., Taylor, L. L., Sarkar, B., Kelland, M., DeLucia, E., Kantola, I., Müller, C., Rau, G., Hansen, J. (2018). Farming with crops and rocks to address global climate, food and soil security. Nature Plants, 4, 138–147. https://doi.org/10.1038/s41477-018-0108-y
Berner, R. A. (2001). GEOCARB III: A revised model of atmospheric CO2 over Phanerozoic time. American Journal of Science, 301, 182–204. https://doi.org/10.2475/ajs.301.2.182
Boulvain, F., Coen-Aubert, M. (2006). A fourth level of Frasnian carbonate mounds along the south side of the Dinant Synclinorium (Belgium). Bulletin de l’Institut Royal des Sciences Naturelles de Belgique, Sciences de la Terre, 76, 31–51.
Braga, D., Candina, J., Eceiza, J., Esgalhado, B., González, D., Marcos, I. (2022). Net-zero Spain: Europe’s Decarbonization Hub. Madrid, Spain: McKinsey & Company.
Bremen, A. M., Strunge, T., Ostovari, H., Spütz, H., Mhamdi, A., Renforth, P., van der Spek, M., Bardow, A., Mitsos, A. (2022). Direct Olivine Carbonation: Optimal Process Design for a Low-Emission and Cost-Efficient Cement Production. Ind. Eng. Chem. Res., 61, 13177–13190. https://doi.org/10.1021/acs.iecr.2c00984
Buckingham, F. L., Henderson, G. M., Holdship, P., Renforth, P. (2022). Soil core study indicates limited CO2 removal by enhanced weathering in dry croplands in the UK. Applied Geochemistry, 147, 105482. https://doi.org/10.1016/j.apgeochem.2022.105482
Bullock, L. A., Alcalde, J., Tornos, F., Fernandez-Turiel, J. -L. (2023). Geochemical carbon dioxide removal potential of Spain. Science of The Total Environment, 867, 161287. https://doi.org/10.1016/j.scitotenv.2022.161287
Bullock, L. A., James, R. H., Matter, J., Renforth, P., Teagle, D. A. H. (2021). Global Carbon Dioxide Removal Potential of Waste Materials from Metal and Diamond Mining. Front. Clim., 3, 694175. https://doi.org/10.3389/fclim.2021.694175
Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). (2005). The 1:5 million scale International Geological Map of Europe and Adjacent Areas (IGME5000).
Campbell, J. S., Foteinis, S., Furey, V., Hawrot, O., Pike, D., Aeschlimann, S., Maesano, C. N., Reginato, P. L., Goodwin, D. R., Looger, L. L., Boyden, E. S., Renforth, P. (2022). Geochemical Negative Emissions Technologies: Part I. Review. Front. Clim., 4, 879133. https://doi.org/10.3389/fclim.2022.879133
Carbon Gap. (2025a). EU Carbon Removal Funding. Brussels, Belgium: Carbon Gap.
Carbon Gap. (2025b). Carbon Removal in Spain – National Policy Overview. Brussels, Belgium: Carbon Gap.
Caroff, M., Coint, N., Hallot, E., Hamelin, C., Peucat, J. -J., Charreteur, G. (2011). The mafic–silicic layered intrusions of Saint-Jean-du-Doigt (France) and North-Guernsey (Channel Islands), Armorican Massif: Gabbro–diorite layering and mafic cumulate–pegmatoid association. Lithos, 125, 675–692. https://doi.org/10.1016/j.lithos.2011.03.019
Caserini, S., Storni, N., Grosso, M. (2022). The Availability of Limestone and Other Raw Materials for Ocean Alkalinity Enhancement. Global Biogeochemical Cycles, 36, e2021GB007246. https://doi.org/10.1029/2021GB007246
Clarkson, M. O., Larkin, C. S., Swoboda, P., Reershemius, T., Suhrhoff, T. J., Maesano, C. N., Campbell, J. S. (2024). A review of measurement for quantification of carbon dioxide removal by enhanced weathering in soil. Frontiers in Climate, Volume 6-2024. https://doi.org/10.3389/fclim.2024.1345224
Climate Change Committee. (2025). The Seventh Carbon Budget. London, UK.: Climate Change Committee.
Committee on Developing a Research Agenda for Carbon Dioxide Removal and Reliable Sequestration, Board on Atmospheric Sciences and Climate, Board on Energy and Environmental Systems, Board on Agriculture and Natural Resources, Board on Earth Sciences and Resources, Board on Chemical Sciences and Technology, Ocean Studies Board, Division on Earth and Life Studies, National Academies of Sciences, Engineering, and Medicine. (2019). Negative Emissions Technologies and Reliable Sequestration: A Research Agenda. Washington, D. C.: National Academy Press. https://doi.org/10.17226/25259
Continental Shelf Department. (2023). Geological Map of the Maltese Islands (Scale 1:10,000).
Dreimanis, A., Kārklin, O. L. (1997). LatviaLatvia. In E. M. Moores (Ed.), Encyclopedia of European and Asian Regional Geology (pp. 498–504). Dordrecht (Netherlands): Springer. https://doi.org/10.1007/1-4020-4495-X_59
Eisaman, M. D. (2024). Pathways for marine carbon dioxide removal using electrochemical acid-base generation. Frontiers in Climate, 6, 1349604. https://doi.org/10.3389/fclim.2024.1349604
Eisaman, M. D., Geilert, S., Renforth, P., Bastianini, L., Campbell, J., Dale, A. W., Foteinis, S., Grasse, P., Hawrot, O., Löscher, C.R., Rau, G. H., Rønning, J. (2023). Assessing the technical aspects of ocean-alkalinity-enhancement approaches. State of the Planet 2-oae2023, 3. https://doi.org/10.5194/sp-2-oae2023-3-2023
Erans, M., Nabavi, S. A., Manović, V. (2020). Carbonation of lime-based materials under ambient conditions for direct air capture. Journal of Cleaner Production, 242, 118330. https://doi.org/10.1016/j.jclepro.2019.118330
European Parliament, Council of the European Union. (2024). Regulation (EU) 2024/3012 of the European Parliament and of the Council of 27 November 2024 establishing a Union certification framework for permanent carbon removals, carbon farming and carbon storage in products. Official Journal of the European Union.
European Scientific Advisory Board on Climate Change. (2025). Scaling up Carbon Dioxide Removals: Recommendations for navigating opportunities and risks in the EU (No. 978-92-9480-694–9). Luxembourg: Publications Office of the European Union. https://doi.org/10.2800/3253650
Flipkens, G., Fuhr, M., Fiers, G., Meysman, F. J. R., Town, R. M., Blust, R. (2023). Enhanced olivine dissolution in seawater through continuous grain collisions. Geochimica et Cosmochimica Acta, 359, 84–99. https://doi.org/10.1016/j.gca.2023.09.002
Foteinis, S., Andresen, J., Campo, F., Caserini, S., Renforth, P. (2022). Life cycle assessment of ocean liming for carbon dioxide removal from the atmosphere. Journal of Cleaner Production, 370, 133309. https://doi.org/10.1016/j.jclepro.2022.133309
Foteinis, S., Campbell, J., Madankan, M., Bullock, L., Valenzuela, J.M., Lezaun, J., Renforth, P. Spain’s realistic carbon dioxide removal potential through ocean alkalinity enhancement. (in review).
Fuhr, M., Geilert, S., Schmidt, M., Wallmann, K. (2021). Kinetics of olivine weathering in seawater: an experimental study, in: Goldschmidt 2021 Abstracts. Presented at the Goldschmidt2021. European Association of Geochemistry, Virtual. https://doi.org/10.7185/gold2021.7375
Geological Survey Department. (1979). Geological Map of Cyprus.
Gerdemann, S. J., O’Connor, W. K., Dahlin, D. C., Penner, L. R., Rush, H. (2007). Ex Situ Aqueous Mineral Carbonation. Environ. Sci. Technol., 41, 2587–2593. https://doi.org/10.1021/es0619253
Harangi, S., Downes, H., Thirlwall, M., Gméling, K. (2007). Geochemistry, Petrogenesis and Geodynamic Relationships of Miocene Calc-alkaline Volcanic Rocks in the Western Carpathian Arc, Eastern Central Europe. Journal of Petrology, 48, 2261–2287. https://doi.org/10.1093/petrology/egm059
Harder, J. (2023). The Cement Industry in Europe at the Crossroads. ZKG Cement Lime Gypsum, 76, 46–55.
Hartmann, J., West, A. J., Renforth, P., Köhler, P., De La Rocha, C. L., Wolf-Gladrow, D. A., Dürr, H. H., Scheffran, J. (2013). Enhanced chemical weathering as a geoengineering strategy to reduce atmospheric carbon dioxide, supply nutrients, and mitigate ocean acidification: ENHANCED WEATHERING. Rev. Geophys., 51, 113–149. https://doi.org/10.1002/rog.20004
Huijgen, W. J. J., Witkamp, G. -J., Comans, R. N. J. (2005). Mineral CO2 Sequestration by Steel Slag Carbo nation. Environ. Sci. Technol., 39, 9676–9682. https://doi.org/10.1021/es050795f
Huijgen, W. J. J., Witkamp, G. -J., Comans, R. N. J. (2006). Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chemical Engineering Science, 61, 4242–4251. https://doi.org/10.1016/j.ces.2006.01.048
Huntzinger, D. N., Gierke, J. S., Kawatra, S .K., Eisele, T. C., Sutter, L. L. (2009). Carbon Dioxide Sequestration in Cement Kiln Dust through Mineral Carbonation. Environ. Sci. Technol., 43, 1986–1992. https://doi.org/10.1021/es802910z
Isometric. (2025). Project Serra da Mantiqueira. London, United Kingdom: Isometric Registry.
Jansen, M. W., Münker, C., Pakulla, J.J., Hasenstab-Dübeler, E., Marien, C. S., Schulz, T., Kirchenbaur, M., Schneider, K. P., Tordy, R., Schmitt, V., Wombacher, F. (2024). Petrogenesis of volcanic rocks from the Quaternary Eifel volcanic fields, Germany: detailed insights from combined trace-element and Sr–Nd–Hf–Pb–Os isotope data. Contributions to Mineralogy and Petrology, 179, 57. https://doi.org/10.1007/s00410-024-02137-w
Kantzas, E. P., Val Martin, M., Lomas, M. R., Eufrasio, R. M., Renforth, P., Lewis, A. L., Taylor, L. L., Mecure, J. -F., Pollitt, H., Vercoulen, P. V., Vakilifard, N., Holden, P. B., Edwards, N. R., Koh, L., Pidgeon, N. F., Banwart, S. A., Beerling, D. J. (2022). Substantial carbon drawdown potential from enhanced rock weathering in the United Kingdom. Nat. Geosci., 15, 382–389. https://doi.org/10.1038/s41561-022-00925-2
Kelemen, P. B., McQueen, N., Wilcox, J., Renforth, P., Dipple, G., Vankeuren, A. P. (2020). Engineered carbon mineralization in ultramafic rocks for CO2 removal from air: Review and new insights. Chemical Geology, 550, 119628. https://doi.org/10.1016/j.chemgeo.2020.119628
Kheshgi, H. S. (1995). Sequestering atmospheric carbon dioxide by increasing ocean alkalinity. Energy, 20, 915–922. https://doi.org/10.1016/0360-5442(95)00035-F
Kozhoukharova, E. (2024). The Precambrian Metamorphic Complex in the Rhodope Massif – A Unified Stratigraphic System. Journal of Agricultural, Earth and Environmental Sciences, 3, 1–9.
Lackner, K. S. (2002). Carbonate Chemistry for Sequestering Fossil Carbon. Annu. Rev. Energy. Environ., 27, 193–232. https://doi.org/10.1146/annurev.energy.27.122001.083433
Lackner, K. S., Butt, D. P., Wendt, C. H. (1997). Progress on binding CO2 in mineral substrates. Energy Conversion and Management, 38, S259–S264. https://doi.org/10.1016/S0196-8904(96)00279-8
Lackner, K. S., Wendt, C., Butts, D. P., Joyce, E. L., Sharps, D. H. (1995). Cabon dioxide disposal in carbonate minerals. Energy, 20, (11), 18. https://doi.org/10.1016/0360-5442(95)00071-N
Larkin, C. S., Andrews, M. G., Pearce, C. R., Yeong, K. L., Beerling, D. J., Bellamy, J., Benedick, S., Freckleton, R. P., Goring-Harford, H., Sadekar, S., James, R. H. (2022). Quantification of CO2 removal in a large-scale enhanced weathering field trial on an oil palm plantation in Sabah, Malaysia. Frontiers in Climate, Volume 4-2022. https://doi.org/10.3389/fclim.2022.959229
Leake, B. E. (1989). The metagabbros, orthogneisses and paragneisses of the Connemara complex, western Ireland. Journal of the Geological Society, 146, 575–596. https://doi.org/10.1144/gsjgs.146.4.0575
Lee Pereira, R., Muangthai, I., Azimi, A., Campbell, J., Delval, M., Foteinis, S., Katish, M., Thonemann, N., Strunge, T., Su, D., Ward, C., Van der Spek, M., Renforth, P. (2025). A Framework for Techno-Economic and Life-Cycle Assessment in Ocean Alkalinity Enhancement. Edinburgh, United Kingdom: Heriot-Watt University. https://doi.org/10.17861/v5j0-xw20
Lezaun, J., Valenzuela, J. M. (2024). Realistic Deployment Scenarios for Ocean Alkalinity Enhancement: Ocean Liming (OL). Kiel, Germany: OceanNETs / GEOMAR Helmholtz Centre for Ocean Research Kiel. https://doi.org/10.3289/oceannets_d6.5_1
Madankan, M., Kantzas, E. P., Espinosa, R. M. E., Vetter, S. H., Koh, L., Smith, P., Beerling, D., Renforth, P. A. Spatio-temporal supply-chain framework for Enhanced Rock Weathering deployment at scale: a UK case study (in review).
Madankan, M., Renforth, P. (2023). An inventory of UK mineral resources suitable for enhanced rock weathering. International Journal of Greenhouse Gas Control, 130, 104010. https://doi.org/10.1016/j.ijggc.2023.104010
Madeddu, S., Priestnall, M., Godoy, E., Kumar, R. V., Raymahasay, S., Evans, M., Wang, R., Manenye, S., Kinoshita, H. (2015). Extraction of Mg(OH)2 from Mg silicate minerals with NaOH assisted with H2O: implications for CO2 capture from exhaust flue gas. Faraday Discuss, 183, 369–387. https://doi.org/10.1039/C5FD00047E
Maesano, C. N., Campbell, J. S., Foteinis, S., Furey, V., Hawrot, O., Pike, D., Aeschlimann, S., Reginato, P. L., Goodwin, D. R., Looger, L. L., Boyden, E. S., Renforth, P. (2022). Geochemical Negative Emissions Technologies: Part II. Roadmap. Front. Clim., 4, 945332. https://doi.org/10.3389/fclim.2022.945332
Masson-Delmotte, V., Zhai, P., Pörtner, H. -O., Roberts, D., Skea, J., Shukla, P. R. (2022). Global Warming of 1.5° C: IPCC Special Report on Impacts of Global Warming of 1.5° C above Pre-industrial Levels in Context of Strengthening Response to Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Cambridge University Press.
Matter, J. M., Stute, M., Snæbjörnsdottir, S. Ó., Oelkers, E. H., Gislason, S. R., Aradottir, E. S., Sigfusson, B., Gunnarsson, I., Sigurdardottir, H., Gunnlaugsson, E., Axelsson, G., Alfredsson, H. A., Wolff-Boenisch, D., Mesfin, K., Taya, D. F. de la R., Hall, J., Dideriksen, K., Broecker, W. S. (2016). Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science, 352, 1312–1314. https://doi.org/10.1126/science.aad8132
Mayes, W. M., Riley, A. L., Gomes, H. I., Brabham, P., Hamlyn, J., Pullin, H., Renforth, P. (2018). Atmospheric CO2 Sequestration in Iron and Steel Slag: Consett, County Durham, United Kingdom. Environ. Sci. Technol., 52, 7892–7900. https://doi.org/10.1021/acs.est.8b01883
McCarten, M., Bayaraa, M., Caldecott, B., Christiaen, C., Foster, P., Hickey, C., Kampmann, D., Layman, C., Rossi, C., Scott, K., Tang, K., Tkachenko, N., Yoken, D. (2021). Global Database of Iron and Steel Production Assets.
McQueen, N., Kelemen, P., Dipple, G., Renforth, P., Wilcox, J. (2020). Ambient weathering of magnesium oxide for CO2 removal from air. Nat Commun, 11, 3299. https://doi.org/10.1038/s41467-020-16510-3
Metz, B., Intergovernmental Panel on Climate Change (Eds.). (2005). IPCC special report on carbon dioxide capture and storage: summary for policymakers and technical summary.
Meysman, F. J. R., Montserrat, F. (2017). Negative CO2 emissions via enhanced silicate weathering in coastal environments. Biol. Lett., 13, 20160905. https://doi.org/10.1098/rsbl.2016.0905
Mitchell, C. (2009). Quarry Fines and Waste, in: Quarries & Mines 2009. London: Ten Alps., 63–67.
Montserrat, F., Renforth, P., Hartmann, J., Leermakers, M., Knops, P., Meysman, F. J. R. (2017). Olivine Dissolution in Seawater: Implications for CO2 Sequestration through Enhanced Weathering in Coastal Environments. Environ. Sci. Technol., 51, 3960–3972. https://doi.org/10.1021/acs.est.6b05942
Motuza, G. (2016). Ultramafic Varėna Suite in the Precambrian crystalline basement of the Southern Lithuania – implications for the origin. Baltica, 29, 93–106. https://doi.org/10.5200/baltica.2016.29.09
Nduagu, E., Björklöf, T., Fagerlund, J., Wärnå, J., Geerlings, H., Zevenhoven, R. (2012). Production of magnesium hydroxide from magnesium silicate for the purpose of CO2 mineralisation – Part 1: Application to Finnish serpentinite. Minerals Engineering, 30, 75–86. https://doi.org/10.1016/j.mineng.2011.12.004
Oppon, E. (2020). Enhanced Rock Weathering Supply Chain Lifecycle Sustainability. Sheffield, United Kingdom: University of Sheffield.
Oschlies, A., Bach, L. T., Rickaby, R. E. M., Satterfield, T., Webb, R., Gattuso, J. -P. (2023). Climate targets, carbon dioxide removal, and the potential role of ocean alkalinity enhancement. State of the Planet 2-oae2023, 1. https://doi.org/10.5194/sp-2-oae2023-1-2023
Oschlies, A., Stevenson, A., Bach, L. T., Fennel, K., Rickaby, R. E., Satterfield, T., Webb, R., Gattuso, J. -P. (2023). Guide to best practices in ocean alkalinity enhancement research. https://doi.org/10.5194/sp-2-oae2023
Oyarzun, R., Cubas, P. (2021). Geotour France 1: Cantal and the Chaîne des Puys (Auvergne); volcanoes “a la carte”. Madrid, Spain: Aula2puntonet.
Peccerillo, A. (2017). The Roman Province. In A. Peccerillo (Ed.), Cenozoic Volcanism in the Tyrrhenian Sea Region (pp. 81-124). Cham.: Springer International Publishing. https://doi.org/10.1007/978-3-319-42491-0_4
Peccerillo, A. (2020). 5 - Campania volcanoes: petrology, geochemistry, and geodynamic significance. In B. De Vivo, H. E. Belkin, G. Rolandi (Eds.), Vesuvius, Campi Flegrei, and Campanian Volcanism (pp. 79–120). Elsevier. https://doi.org/10.1016/B978-0-12-816454-9.00005-5
Précigout, J., Gueydan, F., Garrido, C. J., Cogné, N., Booth-Rea, G. (2013). Deformation and exhumation of the Ronda peridotite (Spain). Tectonics, 32, 1011–1025. https://doi.org/10.1002/tect.20062
Pullin, H., Bray, A. W., Burke, I. T., Muir, D. D., Sapsford, D. J., Mayes, W. M., Renforth, P. (2019). Atmospheric Carbon Capture Performance of Legacy Iron and Steel Waste. Environ. Sci. Technol., 53, 9502–9511. https://doi.org/10.1021/acs.est.9b01265
Ragipani, R., Sreenivasan, K., Anex, R. P., Zhai, H., Wang, B. (2022). Direct Air Capture and Sequestration of CO2 by Accelerated Indirect Aqueous Mineral Carbonation under Ambient Conditions. ACS Sustainable Chem. Eng., 10, 7852–7861. https://doi.org/10.1021/acssuschemeng.1c07867
Rau, G. H. (2008). Electrochemical Splitting of Calcium Carbonate to Increase Solution Alkalinity: Implications for Mitigation of Carbon Dioxide and Ocean Acidity. Environ. Sci. Technol., 42, 8935–8940. https://doi.org/10.1021/es800366q
Rau, G. H. (2011). CO2 Mitigation via Capture and Chemical Conversion in Seawater. Environ. Sci. Technol., 45, 1088–1092. https://doi.org/10.1021/es102671x
Rau, G. H., Carroll, S. A., Bourcier, W. L., Singleton, M. J., Smith, M. M., Aines, R. D. (2013). Direct electrolytic dissolution of silicate minerals for air CO2 mitigation and carbon-negative H2 production. Proc. Natl. Acad. Sci. U.S.A. 110(25), 10095–10100. https://doi.org/10.1073/pnas.1222358110
Reershemius, T., Kelland, M. E., Jordan, J. S., Davis, I. R., D’Ascanio, R., Kalderon-Asael, B., Asael, D., Suhrhoff, T. J., Epihov, D. Z., Beerling, D. J., Reinhard, C. T., Planavsky, N. J. (2023). Initial Validation of a Soil-Based Mass-Balance Approach for Empirical Monitoring of Enhanced Rock Weathering Rates. Environ. Sci. Technol., 57, 19497–19507. https://doi.org/10.1021/acs.est.3c03609
Renforth, P. (2012). The potential of enhanced weathering in the UK. International Journal of Greenhouse Gas Control, 10, 229–243. https://doi.org/10.1016/j.ijggc.2012.06.011
Renforth, P. (2019). The negative emission potential of alkaline materials. Nat Commun, 10, 1401. https://doi.org/10.1038/s41467-019-09475-5
Renforth, P., Baltruschat, S., Peterson, K., Mihailova, B. D., Hartmann, J. (2022). Using ikaite and other hydrated carbonate minerals to increase ocean alkalinity for carbon dioxide removal and environmental remediation. Joule, 6, 2674–2679. https://doi.org/10.1016/j.joule.2022.11.001
Renforth, P., Campbell, J., Foteinis, S., Cosgun, E., Young, J., Strunge, T., Riley, A. L., Mayes, W. M., van der Spek, M. W. (2024). Carbon dioxide removal could result in the use of lower-grade iron ore in a decarbonized net-negative emission steel industry. Journal of Cleaner Production, 468, 142987. https://doi.org/10.1016/j.jclepro.2024.142987
Renforth, P., Henderson, G. (2017). Assessing ocean alkalinity for carbon sequestration: Ocean Alkalinity for C Sequestration. Rev. Geophys., 55, 636–674. https://doi.org/10.1002/2016RG000533
Renforth, P., Jenkins, B. G., Kruger, T. (2013). Engineering challenges of ocean liming. Energy, 60, 442–452. https://doi.org/10.1016/j.energy.2013.08.006
Renforth, P., Manning, D. A. C., Lopez-Capel, E. (2009). Carbonate precipitation in artificial soils as a sink for atmospheric carbon dioxide. Applied Geochemistry, 24, 1757–1764. https://doi.org/10.1016/j.apgeochem.2009.05.005
Renforth, P., Washbourne, C. -L., Taylder, J., Manning, D. A. C. (2011). Silicate Production and Availability for Mineral Carbonation. Environ. Sci. Technol., 45, 2035–2041. https://doi.org/10.1021/es103241w
Renna, M. R., Tribuzio, R. (2011). Olivine-rich Troctolites from Ligurian Ophiolites (Italy): Evidence for Impregnation of Replacive Mantle Conduits by MORB-type Melts. Journal of Petrology, 52, 1763–1790. https://doi.org/10.1093/petrology/egr029
Savov, I., Ryan, J., Haydoutov, I., Schijf, J. (2001a). Late Precambrian Balkan-Carpathian ophiolite — a slice of the Pan-African ocean crust?: geochemical and tectonic insights from the Tcherni Vrah and Deli Jovan massifs, Bulgaria and Serbia. Journal of Volcanology and Geothermal Research, 110, 299–318. https://doi.org/10.1016/S0377-0273(01)00216-5
Savov, I., Ryan, J., Haydoutov, I., Schijf, J. (2001b). Late Precambrian Balkan-Carpathian ophiolite — a slice of the Pan-African ocean crust?: geochemical and tectonic insights from the Tcherni Vrah and Deli Jovan massifs, Bulgaria and Serbia. Journal of Volcanology and Geothermal Research, 110, 299–318. https://doi.org/10.1016/S0377-0273(01)00216-5
Schuiling, R. D., de Boer, P. L. (2010). Coastal spreading of olivine to control atmospheric CO2 concentrations: A critical analysis of viability. Comment: Nature and laboratory models are different. International Journal of Greenhouse Gas Control, 4, 855–856. https://doi.org/10.1016/j.ijggc.2010.04.012
Schuiling, R. D., Krijgsman, P. (2006). Enhanced Weathering: An Effective and Cheap Tool to Sequester CO2. Climatic Change, 74, 349–354. https://doi.org/10.1007/s10584-005-3485-y
Seifritz, W. (1990). CO2 disposal by means of silicates. Nature, 345, 486–486. https://doi.org/10.1038/345486b0
Smith, N. (2013). CO2StoP GIS Database and Map Resources: Assessment of CO₂ Storage Potential in Europe.
Snæbjörnsdóttir, S. Ó., Sigfússon, B., Marieni, C., Goldberg, D., Gislason, S. R., Oelkers, E. H. (2020). Carbon dioxide storage through mineral carbonation. Nature Reviews Earth & Environment, 1, 90–102. https://doi.org/10.1038/s43017-019-0011-8
Spence, E., Cox, E., Pidgeon, N. (2021). Exploring cross-national public support for the use of enhanced weathering as a land-based carbon dioxide removal strategy. Climatic Change, 165, 23. https://doi.org/10.1007/s10584-021-03050-y
Stasiulaitiene, I., Fagerlund, J., Nduagu, E., Denafas, G., Zevenhoven, R. (2011). Carbonation of serpentinite rock from Lithuania and Finland. Energy Procedia, 4, 2963–2970. https://doi.org/10.1016/j.egypro.2011.02.205
Steg, L., Veldstra, J., de Kleijne, K., Kılkış, Ş., Lucena, A. F. P., Nilsson, L. J., Sugiyama, M., Smith, P., Tavoni, M., de Coninck, H., van Diemen, R., Renforth, P., Mirasgedis, S., Nemet, G., Görsch, R., Muri, H., Bertoldi, P., Cabeza, L. F., Mata, É., Novikova, A., Caldas, L. R., Chàfer, M., Khosla, R., Vérez, D. (2022). A method to identify barriers to and enablers of implementing climate change mitigation options. One Earth, 5, 1216–1227. https://doi.org/10.1016/j.oneear.2022.10.007
Stolaroff, J. K., Lowry, G. V., Keith, D. W. (2005). Using CaO- and MgO-rich industrial waste streams for carbon sequestration. Energy Conversion and Management, 46, 687–699. https://doi.org/10.1016/j.enconman.2004.05.009
Sun, X., Alcalde, J., Bakhtbidar, M., Elío, J., Vilarrasa, V., Canal, J., Ballesteros, J., Heinemann, N., Haszeldine, S., Cavanagh, A., Vega-Maza, D., Rubiera, F., Martínez-Orio, R., Johnson, G., Carbonell, R., Marzan, I., Travé, A., Gomez-Rivas, E. (2021). Hubs and clusters approach to unlock the development of carbon capture and storage – Case study in Spain. Applied Energy, 300, 117418. https://doi.org/10.1016/j.apenergy.2021.117418
Tkachenko, N., Tang, K., McCarten, M., Reece, S., Kampmann, D., Hickey, C., Bayaraa, M., Foster, P., Layman, C., Rossi, C., Scott, K., Yoken, D., Christiaen, C., Caldecott, B. (2023). Global database of cement production assets and upstream suppliers. Scientific Data, 10, 696. https://doi.org/10.1038/s41597-023-02599-w
Tromans, D. (2008). Mineral comminution: Energy efficiency considerations. Minerals Engineering 21, 613–620. https://doi.org/10.1016/j.mineng.2007.12.003
Tubiello, F. N., Conchedda, G., Casse, L., Pengyu, H., Zhongxin, C., De Santis, G., Fritz, S., Muchoney, D. (2023). Measuring the world’s cropland area. Nature Food, 4, 30–32. https://doi.org/10.1038/s43016-022-00667-9
UEPG. (2025). Aggregates Europe [WWW Document]. https://www.aggregates-europe.eu/facts-figures/figures/
UK Government. (2021). Net Zero Strategy: Build Back Greener. London, UK.: HM Government.
Ulrych, J., Štěpánková-Svobodová, J. (2014). Cenozoic alkaline volcanic rocks with carbonatite affinity in the Bohemian Massif: Their sources and magma generation. Mineralia Slovaca, 46, 45–58.
Wang, X., Maroto-Valer, M. M. (2011). Dissolution of serpentine using recyclable ammonium salts for CO2 mineral carbonation. Fuel, 90, 1229–1237. https://doi.org/10.1016/j.fuel.2010.10.040
Washbourne, C. -L., Lopez-Capel, E., Renforth, P., Ascough, P. L., Manning, D. A. C. (2015). Rapid Removal of Atmospheric CO2 by Urban Soils. Environ. Sci. Technol., 49, 5434–5440. https://doi.org/10.1021/es505476d
Webb, R. M., Silverman-Roati, K., Gerrard, M. B. (2021). Removing Carbon Dioxide Through Seaweed Cultivation: Legal Challenges and Opportunities. New York, NY.: Sabin Center for Climate Change Law, Columbia Law School.
Wilson, S. A., Dipple, G. M., Power, I. M., Thom, J. M., Anderson, R. G., Raudsepp, M., Gabites, J. E., Southam, G. (2009). Carbon Dioxide Fixation within Mine Wastes of Ultramafic-Hosted Ore Deposits: Examples from the Clinton Creek and Cassiar Chrysotile Deposits, Canada. Economic Geology, 104, 95–112. https://doi.org/10.2113/gsecongeo.104.1.95
Wilson, S. A., Harrison, A. L., Dipple, G. M., Power, I. M., Barker, S. L. L., Ulrich Mayer, K., Fallon, S. J., Raudsepp, M., Southam, G. (2014). Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. International Journal of Greenhouse Gas Control, 25, 121–140. https://doi.org/10.1016/j.ijggc.2014.04.002
Xi, F., Davis, S. J., Ciais, P., Crawford-Brown, D., Guan, D., Pade, C., Shi, T., Syddall, M., Lv, J., Ji, L., Bing, L., Wang, J., Wei, W., Yang, K. -H., Lagerblad, B., Galan, I., Andrade, C., Zhang, Y., Liu, Z. (2016). Substantial global carbon uptake by cement carbonation. Nature Geosci, 9, 880–883. https://doi.org/10.1038/ngeo2840
Zhang, J., Zhang, R., Geerlings, H., Bi, J. (2010). A Novel Indirect Wollastonite Carbonation Route for CO2 Sequestration. Chem. Eng. Technol., 33, 1177–1183. https://doi.org/10.1002/ceat.201000024
NOTES
* The author would like acknowledge funding from the European Union’s Horizon 2020 Research and Innovation Program under grant 869357 (project OceanNETs) and through the European Union’s Horizon Europe research and innovation programme under grant agreement No. 101081362 (project SEAO2-CDR). Although the perspective articulated here represents the views of the author and not necessarily that of the projects.
** Research Centre for Carbon Solutions, Heriot-Watt University, Edinburgh, EH14 4AS, United Kingdom.
